Pediatric Crohn’s disease (pCD) is a rare, inflammatory bowel disease characterized by severe, chronic inflammation of the intestinal wall or any portion of the gastrointestinal tract. Crohn’s Disease is most common in individuals between 15-35, with approximately 25% diagnosed by age 20. The total prevalent cases in the G7 countries are anticipated to rise to 82,519 by 2030 at a CAGR of 2.1% for the study period (2017-2030). Despite the availability of current therapeutics, there remains the unmet need for more effective treatment to reduce the recurrence rate which is quite high. Emerging drugs like Mirikizumab (Eli Lilly and Company), Stelara (Janssen Research & Development, LLC), Entyvio(Takeda Oncology), and others are likely capable to eliminate the current challenges and also have the potential to drive the market size of pediatric Crohn’s Disease (pCD). The reduced cost of biosimilars relative to Pediatric Crohn’s disease can drive market competition, contributing to budget sustainability and improving patient access to biologic treatments. 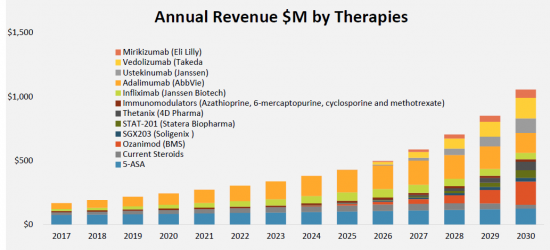 The most widely used drugs in Crohn’s Disease are corticosteroids, immunosuppressants, and biologicals (anti-TNF, anti-interleukins, and anti-integrins). The landscape of pediatric Crohn’s disease (CD) is rapidly changing. Therapeutic advances seen in the adult arena are rapidly adopted by pediatric gastroenterologists and evaluated in both controlled studies and real-world experience. Additionally, pediatrics is leading the way as in a primary therapy which uses regimens including diet specific carbohydrate (SCD) and exclusive enteral nutrition (EEN). With the advent of the market entry of anticancer necrosis factor alpha (TNF-?) inhibitors, the market landscape has changed with more effective drugs with the potential for prolonged remission. Read More: https://www.giiresearch.com/report/mell1043374-pediatric-crohns-disease-pcd-primary-research-kols.html
The most widely used drugs in Crohn’s Disease are corticosteroids, immunosuppressants, and biologicals (anti-TNF, anti-interleukins, and anti-integrins). The landscape of pediatric Crohn’s disease (CD) is rapidly changing. Therapeutic advances seen in the adult arena are rapidly adopted by pediatric gastroenterologists and evaluated in both controlled studies and real-world experience. Additionally, pediatrics is leading the way as in a primary therapy which uses regimens including diet specific carbohydrate (SCD) and exclusive enteral nutrition (EEN). With the advent of the market entry of anticancer necrosis factor alpha (TNF-?) inhibitors, the market landscape has changed with more effective drugs with the potential for prolonged remission. Read More: https://www.giiresearch.com/report/mell1043374-pediatric-crohns-disease-pcd-primary-research-kols.html