
The demand for in-vitro diagnostic products due to the COVID-19 pandemic is expected to increase mainly due to factors such as a sharp rise in market demand for PCR, NGS, serology based rapid-test products, the supportive regulatory landscape for product development & commercialization, and a sharp rise in target patient population. These factors have prompted market players to improve and strengthen their current manufacturing and distribution capabilities as well as to focus on product commercialization & upgrades.
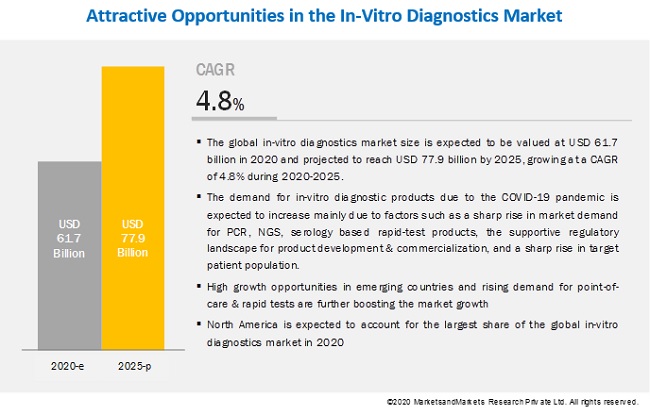
The global in-vitro diagnostics market size is expected to be valued at USD 61.7 billion in 2020 and projected to reach USD 77.9 billion by 2025, growing at a CAGR of 4.8% during 2020-2025.
Download PDF Brochure @
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=55486975
The PCR segment is expected to hold the largest share of the market in 2020 H1
Based on technology, the in-vitro diagnostics market is segmented into PCR, NGS, ELISA, rapid-tests, clinical chemistry, hematology, hemostasis, urinalysis, microbiology testing, and others. The PCR segment is expected to hold the largest share of the global in-vitro diagnostics market in 2020 – H1. Factors such as the increasing patient emphasis on effective & early patient screening, continued commercialization of novel COVID screening platforms by major players, early efforts of key players to address supply chain bottlenecks, and easy availability of controls & standards are driving the growth of this segment.
The US to account for the largest share of the in-vitro diagnostics market in 2020 H1
The US is expected to account for the largest share of the in-vitro diagnostics market in 2020 – H1, followed by Europe. This can primarily be attributed to the continuous commercialization of innovative diagnostic products coupled with ongoing advancements in the field of gene & immunoassay based products, the recent discovery of genetic biomarkers & their clinical role in immunoassay testing, supportive government policies & their emphasis on novel product development, and the significant expansion of target patient population.
Recent Developments (2019-2020):
+ In March 2020, Abbott expanded their manufacturing capacity to offer 5 million ID now test kits per month from April onwards
+ In April 2020, Hologic has strengthened its production line for CDx units to provide 600K test kits per month
+ In March 2020, IDT has announced its capabilities to offer 5 million diagnostic test kits from April 1st week for COVID screening
+ In March 2020, BARDA (US) has provided funding of USD 0.7 million to Hologic to develop rapid diagnostic kits for COVID
Request for Sample Pages @
https://www.marketsandmarkets.com/requestsampleNew.asp?id=55486975
Key Market Players
Major players covered include Bio-Rad Laboratories, Inc. (US), F. Hoffman-La Roche Ltd. (Switzerland), QIAGEN NV (Netherlands), Thermo Fisher Scientific Inc. (US), bioMarieux S.A., Immucor, Inc. (US), Illumina, Inc. (US), Luminex Corporation (US), CareDx (US), Becton, Dickinson and Company (US), Hologic (US), GenDx (Netherlands), Biofortuna (UK), Takara Bio, and BAG Healthcare
An analysis of the market developments between December 2019 to April 2020 revealed that product commercialization, developmental collaborations, and product distribution partnerships were adopted by market players to maintain a competitive position in the in-vitro diagnostics market to mitigate the COVID-19 impact. Product commercialization was the most widely adopted growth strategy.